In clinical chemistry, estimation of serum electrolytes is also an essential, as their concentrations affect variety of biological functions. The simple instrumentation and ease of operation makes it again a desirable addition to the lab floor to complete the clinical biochemistry profile.
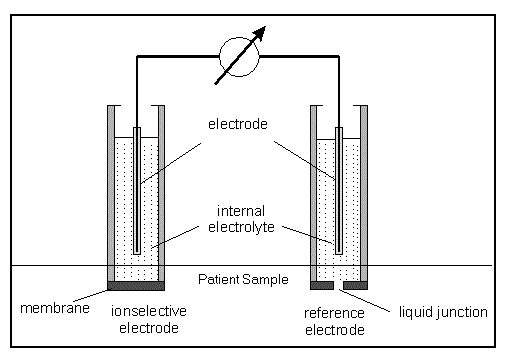
Principle of Ion Selective Electrode (ISE)
This technology involves use of a pH meter or an ion selective probe to identify and quantify each single dissolved ion in the solution. It works on the principle of Nernst equation and can measure both positive as well as negative ions in a solution without being affected by any interference of other dissolved components.
The major advantages include ease of operation and cost-effectiveness as compared to other another analytical platforms which can also be used. Indirect ISE, majorly used in the chemistry analyzers, measures on a total plasma sample (or serum) that has been diluted with a large volume of diluent wherein the plasma and erythrocytes are separated by centrifugation. Due to dilution, this method measures the mean concentration in plasma, i.e., the weighted average between the concentration in the electrolyte - containing water part and in the electrolyte - free protein/lipid part. The concentration is calculated by multiplying the result with the dilution factor. The reported result depends on the content of solids in the sample.
In Thyrocare, the biochemistry section deals with analysis of multiple parameters that aid in diagnosis of health of organs like heart, liver, kidney, etc; the analysis of serum electrolytes also becomes an essential to generate a complete report. The analyzer which aids in this analysis is the ADVIA 2400, which measures Na+ and Cl- in serum.


